Science: 2013: CBSE: [Delhi]: Set – I
To Access the full content, Please Purchase
-
Q1
How many vertical columns are there in the modern periodic table and what are they called?
Marks:1View AnswerAnswer:
Modern periodic table has 18 vertical columns and they are called as ‘Groups’.
-
Q2
What is speciation?
Marks:1View AnswerAnswer:
Speciation is as an evolutionary process which involves the formation of new species from the existing species.
-
Q3
Why should biodegradable and non- biodegradable wastes be discarded in two separate dustbins?
Marks:1View AnswerAnswer:
Biodegradable and non- biodegradable wastes should be discarded in two separate dustbins for proper management of wastes. Biodegradable and non- biodegradable wastes differ in their natural composition and hence, are degraded in different ways. Biodegradable wastes are degraded naturally by the action of microorganisms present in the soil, while non-biodegradable wastes are melted and reused.
-
Q4
“The chromosomes number of the sexually reproducing parents and their offspring is the same.” Justify this statement.
Marks:2View AnswerAnswer:
Somatic cells of human beings have 23 pairs of chromosomes. The number of chromosomes in the gametes, arising from the germ cells, is half the number of chromosomes in the somatic cells of the body. When these gametes combine during fertilisation, the original chromosome number is restored in the zygote. The zygote develops into complete individual having same chromosome number as parent.
-
Q5
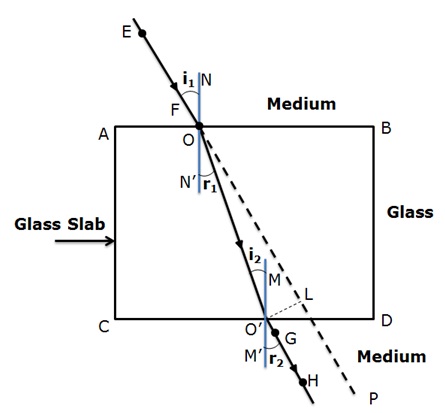
“A ray of light incident on a rectangular glass slab immersed in any medium emerges parallel to itself.” Draw labelled ray diagram to justify the statement.
Marks:2View AnswerAnswer:
Below given is the ray diagram in which the ray EF is the incident ray and GH is the emergent ray which is parallel to the incident ray.
-
Q6
We often observe domestic waste decomposing in the bylanes of residential colonies. Suggest ways to make people realise that the improper disposal of waste is harmful to the environment.
Marks:2View AnswerAnswer:
Improper disposal of waste is harmful to the environment as:
- Improper disposal of waste serves as breeding ground for mosquitoes, which leads to the spread of diseases.
- Improper disposal of waste also leads to release of harmful gases into the environment.
-
Q7
List and explain any two advantages associated with water harvesting at community level.
Marks:2View AnswerAnswer:
The two advantages associated with water harvesting at community level are:
- It helps to overcome the scarcity of water.
- It helps in reducing the effects of droughts and floods.
-
Q8
Write the name and the structural formula of the compound formed when ethanol is heated at 443 K with excess of conc. H2SO4. State the role of conc. H2SO4 in this reaction. Write chemical equation for the reaction.
Marks:3View AnswerAnswer:
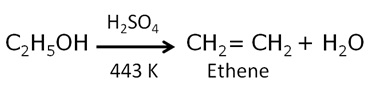
The main product formed during the reaction is Ethene along with water.The structure of ethene is as follows
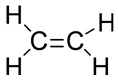
In this reaction, H2SO4 acts as a dehydrating agent. Therefore, ethanol undergoes dehydration i.e., loses a water molecule to form ethene.
-
Q9
Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their (i) physical properties, and (ii) chemical properties.
Marks:3View AnswerAnswer:
Homologous series of carbon compounds are so called because in such a series of compounds, the same functional group is present which decides the properties of the carbon compound irrespective of the length of the carbon chain. The two consecutive members of a homologous series of alcohols are CH3OH and C2H5OH
Name
Part that determines physical properties
Part that determines chemical properties
CH3OH
CH3
OH
C2H5OH
C2H5
OH
-
Q10
Given below are some elements of the modern periodic table:
4Be, 9F, 14Si, 19K, 20Ca
(i) Select the element that has one electron in the outermost shell and write its electronic configuration.
(ii) Select two elements that belong to the same group. Give reason for your answer.
(iii) Select two elements that belong to the same period. Which one of the two has bigger atomic size?
Marks:3View AnswerAnswer:
(i)Electronic configuration of 4Be =2,2
Electronic configuration of 9F =2,7
Electronic configuration of 14Si =2,8,4
Electronic configuration of 19K =2,8,8,1
Electronic configuration of 20Ca =2,8,8,2
(i)The electronic configuration shows 19K has one electron in the outermost shell.
(ii) Since, 4Be and 20Ca both have two electrons in the valence shell, therefore, they belongs to same group i.e. Group-2.
4Be – 2, 2
20Ca- 2, 8, 8, 2
(iii) 9F and 4Be belongs to the same period, Period 2 because both of them have same number of shells.
9F – 2, 7
4Be – 2, 2
4Be has bigger atomic size then 9F because the atomic radius decreases as we move from left to right due to increase in nuclear charge which pulls the electrons closer to the nucleus.



