Science: 2013: CBSE: [Delhi]: Set – II
To Access the full content, Please Purchase
-
Q1
How many horizontal rows are there in the modern periodic table and what are they called?
Marks:1View AnswerAnswer:
Modern periodic table has 7 horizontal rows and they are called as ‘Periods’.
-
Q2
List any two factors that could lead to speciation?
Marks:1View AnswerAnswer:
The two factors which could lead to speciation are natural selection and genetic drift.
-
Q3
Mention one negative effect of our affluent life style on the environment.
Marks:1View AnswerAnswer:
One negative effect of our affluent life style on the environment is global warming. It has resulted due to excess release of greenhouse gases from vehicles, industries in the environment etc.
-
Q4
Mention the two functions of human testis.
Marks:2View AnswerAnswer:
The two functions of human testis are:
- It produces male gametes (sperm).
- It secretes testosterone which is responsible for the development of secondary sexual characteristics in males.
-
Q5
Every one of us can do something to reduce our consumption of various natural resources. List four such activities based on 3-R approach.
Marks:2View AnswerAnswer:
3R strategy is an approach to reduce the amount of solid waste. It stands for reduce, recycle and reuse. Four activities based on 3-R approach are:
- Avoid the use of plastic or polythene bags as they are non-biodegradable. So, they pollute the environment.
- Prefer the use of paper bags as they can be recycled and reused.
- Switch off the electrical appliances when not in use in order to reduce the electricity consumption.
- Use the reverse side of paper and envelope in order to save paper.
-
Q6
“A ray of light incident on a rectangular glass slab immersed in any medium emerges parallel to itself.” Draw labelled ray diagram to justify the statement.
Marks:2View AnswerAnswer:
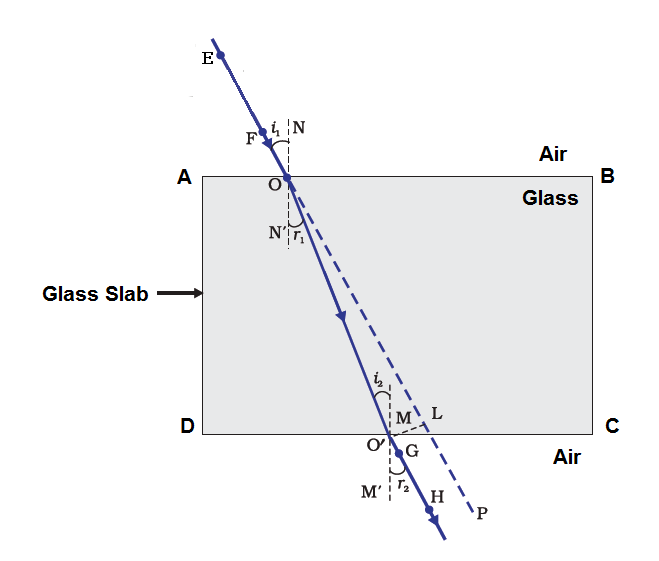
EO is the incident ray and O’H is the emergent ray, which is parallel to the incident ray.
-
Q7
We often observe domestic waste decomposing in the bylanes of residential colonies. Suggest ways to make people realise that the improper disposal of waste is harmful to the environment.
Marks:2View AnswerAnswer:
Improper disposal of waste is harmful to the environment as:
- Improper disposal of waste serves as breeding ground for mosquitoes, which leads to the spread of diseases.
- Improper disposal of waste also leads to release of harmful gases into the environment.
-
Q8
Name the oxidising agent used for the conversion of ethanol to ethanoic acid. Distinguish between ethanol and ethanoic acid on the basis of (i) litmus paper, (ii) reaction with sodium carbonate.
Marks:3View AnswerAnswer:
The oxidising agent used for the conversion of ethanol to ethanoic acid is alkaline potassium permanganate or acidified potassium dichromate.
The difference between ethanol and ethanoic is as follows:
- Litmus paper
Ethanol does not show any change in the colour of litmus paper whereas ethanoic acid turns blue litmus paper red as it is acidic in nature.
- Sodium carbonate
Ethanol does not give any reaction with sodium carbonate.
Ethanoic acid reacts with sodium carbonate to form sodium ethanoate, water and carbon dioxide gas.
-
Q9
(a) Differentiate between alkanes and alkenes. Name and draw the structure of one member of each.
(b) Alkanes generally burn with clean flame. Why?
Marks:3View AnswerAnswer:
(a) The difference between alkanes and alkenes is as follows:
S.No.
Alkanes
Alkenes
The general formula for alkanes is CnH2n+2, where n=1, 2, 3,..
The general formula for alkenes is CnH2n, where n= 2, 3, 4,..
They are the hydrocarbons that consist of only single covalent bonds i.e.(C–C)
They are the hydrocarbons that contain at least one double covalent bond i.e. (C=C)
(b) Alkanes (saturated hydrocarbons) will generally burn with a clean flame because saturated compounds contain comparatively less percentage of carbon which get completely oxidised by the oxygen present in the air. On the other hand, the percentage of carbon in unsaturated compounds is more and it requires more oxygen to get completely oxidised. Hence there is insufficient supply of oxygen for combustion of unsaturated hydrocarbons.
-
Q10
Given below are some elements of the modern periodic table:
4Be, 9F, 14Si, 19K, 20Ca
(i) Select the element that has one electron in the outermost shell and write its electronic configuration.
(ii) Select two elements that belong to the same group. Give reason for your answer.
(iii) Select two elements that belong to the same period. Which one of the two has bigger atomic size?
Marks:3View AnswerAnswer:
(i)Electronic configuration of 4Be =2,2
Electronic configuration of 9F =2,7
Electronic configuration of 14Si =2,8,4
Electronic configuration of 19K =2,8,8,1
Electronic configuration of 20Ca=2,8,8,2
The electronic configuration shows19K has one electron in the outermost shell.
(ii) Since, 4Be and 20Ca both have two electrons in the valence shell, therefore, they belongs to same group i.e. Group-2.
4Be – 2, 2
20Ca- 2, 8, 8, 2
(iii) 9F and 4Be belongs to the same period, Period 2 because both of them have same number of shells.
9F – 2, 7
4Be – 2,2
4Be has bigger atomic size then 9F because the atomic radius decreases as we move from left to right due to increase in nuclear charge which pulls the electrons closer to the nucleus.



