Science: 2013: CBSE: [Delhi]: Set – III
To Access the full content, Please Purchase
-
Q1
Write two reasons responsible for late discovery of noble gases?
Marks:1View AnswerAnswer:
The two reasons for the late discoveries of noble gases are-
(a) They are very inert.
(b) They are present in extremely low concentrations in our environment.
-
Q2
List any two measures that you suggest for better management of water resources.
Marks:1View AnswerAnswer:
The two measures for better management of water resources are:
- Rain water harvesting
- Construction of Dams
-
Q3
“Cell division is a type of reproduction in unicellular organism”. Justify.
Marks:1View AnswerAnswer:
Cell division is a type of reproduction in unicellular organism because many unicellular organisms split into two identical halves during cell division leading to the creation of new organisms.
-
Q4
(a) Trace the path of sperms from where they are produced in human body to the exterior.
(b) Write the functions of secretions of prostate gland and seminal vesicles in humans.
Marks:2View AnswerAnswer:
(a). Testis – Epididymis- Vas deferens- Urethra – Penis.
(b). Seminal Vesicle: It secretes seminal fluid which is thick, clear and contains mucus, amino acids, fructose to protect and nourish the sperms.
Prostate gland: It secretes a slightly alkaline fluid (prostate fluid) that carries sperm to the urethra of males
-
Q5
A ray of light falls normally on the surface of a transparent glass slab. Draw a ray diagram to show its path and also mark angle of incidence and angle of emergence.
Marks:2View AnswerAnswer:
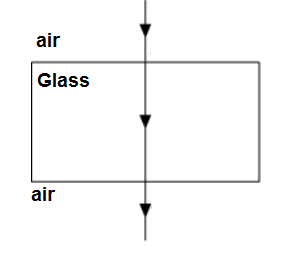
As the incident light is normal to the surface thus the incidence angle will be zero which will correspond to zero degree refracted angle and zero degree emergent angle.
-
Q6
List and explain any two advantages associated with water harvesting at community level.
Marks:2View AnswerAnswer:
The two advantages associated with water harvesting at community level are:
- It helps to overcome the scarcity of water.
- It helps in reducing the effects of droughts and floods.
-
Q7
We often observe domestic waste decomposing in the bylanes of residential colonies. Suggest ways to make people realise that the improper disposal of waste is harmful to the environment.
Marks:2View AnswerAnswer:
Improper disposal of waste is harmful to the environment as:
- Improper disposal of waste serves as breeding ground for mosquitoes, which leads to the spread of diseases.
- Improper disposal of waste also leads to release of harmful gases into the environment.
-
Q8
What happen when:
(a) ethanol is burnt in air
(b) ethanol is heated with excess conc. H2SO4 at 443 K.
(c) a piece of sodium is dropped into ethanol?
Marks:3View AnswerAnswer:
(a) When Ethanol burns in air it gives CO2 and H2O.
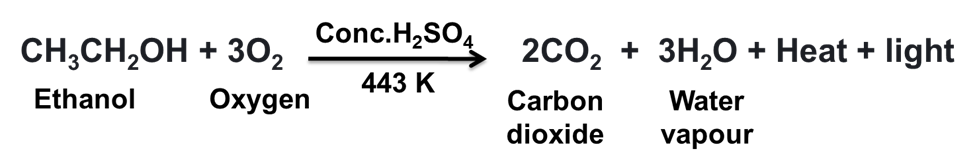
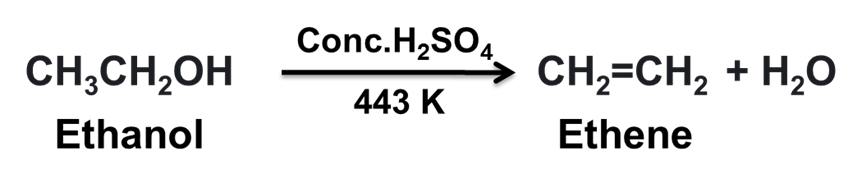
(b) When ethanol is heated with the excess of conc. H2SO4, dehydration of ethanol takes place leading to the formation of ethene.
(c) When sodium reacts with ethanol sodium ethoxide and hydrogen gas is formed.
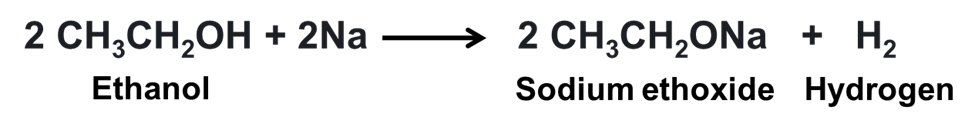
-
Q9
Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their (i) physical properties, and (ii) chemical properties.
Marks:3View AnswerAnswer:
Homologous series of carbon compounds are so called because in such a series of compounds, the same functional group is present which decides the properties of the carbon compound irrespective of the length of the carbon chain. The two consecutive members of a homologous series of alcohols are CH3OH and C2H5OH
Name
Part that determines physical properties
Part that determines chemical properties
CH3OH
CH3
OH
C2H5OH
C2H5
OH
-
Q10
The elements Li, Na and K, each having one valence electron, are in period 2,3 and 4 respectively of modern periodic table.
(i) In which group of the periodic table should they be?
(ii) Which one of them is least reactive?
(iii) Which one of them has the largest atomic radius? Give reason in justify your answer in each case.
Marks:3View AnswerAnswer:
Electronic configuration of Li = 2, 1
Electronic configuration of Na = 2, 8, 1
Electronic configuration of K = 2, 8, 8, 1
(i) As all of them have only one electron in their valence shell they belong to Group 1.
(ii) Li is the least reactive among them due to its smallest size as it has only 2 shells.
(iii) K has the largest atomic radius among the three because it has maximum number of shells. Higher the number of shells, larger is the atomic radius.



