Science 2015 CBSE [All India] Set II
To Access the full content, Please Purchase
-
Q1
Write the number of covalent bonds in the molecule of propane, C3H8.
Marks:1View AnswerAnswer:
There are ten covalent bonds in a molecule of propane.
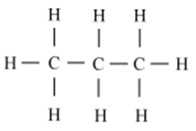
-
Q2
Where is DNA found in a cell?
Marks:1View AnswerAnswer:
DNA is found in the nucleus of a cell.
-
Q3
The first trophic level in a food chain is always a green plant. Why?
Marks:1View AnswerAnswer:
The first trophic level in a food chain is always a green plant because they are the producers. They convert the energy of the sun into a usable form of energy for the next trophic level.
-
Q4
The absolute refractive indices of water and glass are 4/3 and 3/2 respectively. If the speed of light in glass is 2 x 108 m/s, calculate the speed of light in (i) vacuum, (ii) water
Marks:2View AnswerAnswer:
-
Q5
We often observe domestic waste decomposing in the bylanes of our homes. List four ways to make the residents aware that the improper disposal of waste is harmful to the environment and also for their own health.
Marks:2View AnswerAnswer:
The four ways to make the residents aware that the improper disposal of waste is harmful to the environment and also for their own health are:
a) The waste serves as a breeding ground for mosquitoes that spread various diseases.
b) It releases harmful gases that make the environment unclean and unhygienic for all living organism.
c) It flows to water bodies with rain water and harms the aquatic organisms present in it.
d) It reduces the soil fertility and diminishes the beauty of the area.
-
Q6
List any two advantages associated with water stored in the ground.
Marks:2View AnswerAnswer:
The two advantages associated with water stored in the ground are:
- Water does not get contaminated by pollutants.
- Water does not get evaporated.
-
Q7
What is meant by homologous series of carbon compounds? Classify the following carbon compounds into two homologous series and name them.
C3H4, C3H6, C4H6, C4H8, C5H8, C5H10
Marks:3View AnswerAnswer:
Organic compounds with similar general formula belong to a series called homologous series. Alkanes, alkenes, alkynes etc. forms such series and each member differ from its previous member by CH2.
The given compounds are classified as:
Alkenes: C3H6 (propene), C4H8 (butene), C5H10 (pentene)
Alkynes: C3H4 (propyne), C4H6 (butyne), C5H8 (pentyne)
-
Q8
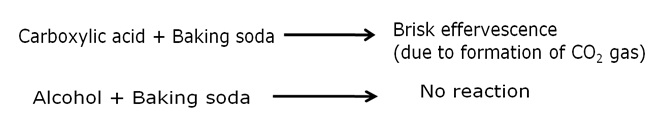
List two tests for experimentally distinguishing between an alcohol and a carboxylic acid and describe how these tests are performed.
Marks:3View AnswerAnswer:
Two tests for experimentally distinguishing between an alcohol and a carboxylic acid are as follows:
a) Baeyer's reagent test: When alcohol is treated with few drops of a 5% alkaline potassium permanganate solution and kept in a hot water bath, the colour of potassium permanganate disappears. On the other hand, carboxylic acids do not show such reaction.
b) Sodium hydrogen carbonate test: Carboxylic acid on reaction with a solution of baking soda gives brisk effervescences due to evolution of carbon dioxide gas. When this gas is passed in lime-water, the later turns milky. Whereas, alcohol do not give effervescence with baking soda solution.
-
Q9
The elements 4Be, 12Mg and 20Ca, each having two valence electrons in their valence shells, are in periods 2, 3 and 4 respectively of the modern periodic table. Answer the following questions associated with these elements, giving reason in each case:
(a) In which group should they be?
(b) Which one of them is least reactive?
(c) Which of them has the largest atomic size?
Marks:3View AnswerAnswer:
(a) All the three elements have a valency of 2. Therefore, they should belong to second group of the periodic table.
(b) Reactivity increases down the group due to increase in size. Therefore, 4Be is least reactive among the three elements.
(c) Atomic size increases down the group due to addition of new shell. Therefore, 20Ca has the largest size among the three elements.
-
Q10
Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of an element is calculated on the basis of its atomic number.
Marks:3View AnswerAnswer:
In the modern periodic table, elements are arranged in 18 vertical columns called groups and 7 horizontal rows called periods. Elements with similar electronic configurations are placed in the same column. Number of valence electrons decides the group of the element and number of shells decides the period of the element.
The valency of an element is determined in the following manner:
a) If the number of electrons in the valence shell is less than 3, then the same number of electrons will represent the valency of that element.
b) If the number of valence electrons is either equal to or greater than 4, then that number is subtracted from 8 to obtain the valency of that element.An element of atomic number 16 has the electronic configuration: 2, 8, 6
It has six valence electrons and three shells. So, its valency is two.
It is positioned in Period 3 and Group 16.



