Science: 2017:CBSE: [All India]:Set–III
To Access the full content, Please Purchase
-
Q1
Write the molecular formula of the 2nd and 3rd member of the homologous series where the first member is ethyne.
Marks:1View AnswerAnswer:
The molecular formulas of 2nd and 3rd member of this series are C3H4 (propyne) and C4H6 (butyne) respectively.
-
Q2
Why is variation important for a species?
Marks:1View AnswerAnswer:
Variation is important for the survival of species as it helps species to adapt to the changing environmental conditions.
-
Q3
In the following food chain, 20,000 J of energy was available to the plants. How much energy would be available to man in this food chain?
Marks:1View AnswerAnswer:
According to ten percent law of energy, only 10% energy is transferred from one trophic level to another. Thus, if 20, 000 J of energy is available to plants, then only 2000 J of energy will be available to sheep and 200 J of energy will be available to man in the given food chain.
-
Q4
Given that the object is placed at a distance 15 cm from lens which means that the object is placed between the optical centre and infinity. The ray diagram for the given case is as follows:
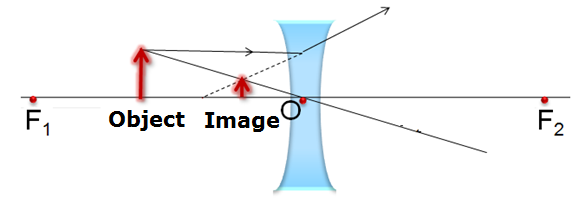
Four characteristics of the image formed are,
(i) The image is formed on the same side as that of the object.
(ii) The image formed is virtual.
(iii) The image formed is erect.
(iv) The image formed is diminished.
Verify these characteristics.
Marks:2View AnswerAnswer:
Given that the object is placed at a distance 15 cm from lens.
i.e, object distance (u) = -15 cm
Focal length of concave lens, f = -30 cm
Image distance (v) =?
By lens formula we know that
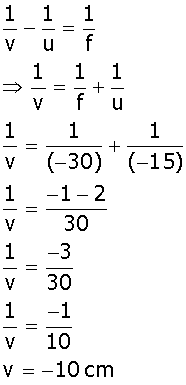
Four characteristics of the image formed are,
(i) Position: The value of (v) shows that the image is formed at a distance of 10 cm from the optical center.
(ii) Nature: The negative sign of the (v) shows the image formed is virtual and erect.
(iii) Size of the image:
For lens magnification,
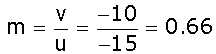
As the value of magnification is less than 1. Hence the image formed is diminished.
(iv) The positive sign of magnification (m) shows that the image is formed above the principal axis.
-
Q5
You being an environmentalist are interest in contributing towards the conservation of natural resources. List four activities that you can do on your own.
Marks:2View AnswerAnswer:
Four activities that we can do on our own are:
- Use of efficient engines for complete combustion of fuels.
- Car pool for same destination.
- Avail the facility of public transport like metro trains.
- Recycle paper bags, use both sides of paper, envelope etc.
-
Q6
Why are coal and petroleum categorized as natural resources? Give a reason as to why they should be used judiciously.
Marks:2View AnswerAnswer:
A natural resource is anything that occurs naturally in the environment and can be used by living organisms. Coal and petroleum are fossil fuels. Fossil fuels are formed naturally from the remains of dead animals and plants which have been subjected to various biological and geological processes.
Coal and petroleum are exhaustible or non-renewable natural resources, i.e., finite quantity of these resources are available in nature. If we continue to use these exhaustible resources at the same rate, nature would not be able to meet our demands and these resources would disappear. Hence, in order to maintain the continuity of these resources, it is necessary to use them judiciously.
-
Q7
Distinguish between esterification and saponification reactions with the help of chemical equation for each. State one use of each (i) esters, and (ii) saponification process.
Marks:3View AnswerAnswer:
Esterification
Saponification
Carboxylic acids react with alcohol in the presence of an acid catalyst to give an ester. This reaction is called the esterification reaction.
It is reverse of esterification reaction.It is the process of hydrolysis of an ester(fats) in the presence of Conc. NaOH to form soap
(i) Use of ester: Esters are used as flavouring agents.
(ii) Use of saponification process: Saponification processis used in making soaps.
-
Q8
Write the structural formula of ethanol. What happens when it is heated with excess of conc. H2SO4 at 443K? Write the chemical equation for the reaction stating the role of conc. H2SO4 in this reaction.
Marks:3View AnswerAnswer:
When ethanol is heated at 443K with excess of conc. H2SO4,ethene(C2H4) is obtained.In this reaction, sulphuric acid act as a dehydrating agent.
-
Q9
What is periodicity in properties of elements with reference to the Modern Periodic Table?Why do all the elements of the same group have similar properties? How does the tendency of elements to gain electrons change as we move from left to right in a period? State the reason of this change.
Marks:3View AnswerAnswer:
According to Modern Periodic Law, physical and chemical properties of elements are periodic functions of their atomic numbers.
Elements belonging to same group have same number of electrons in the valence shell. So, they show similar properties.
As we move from left to right in a period, tendency of elements to gain electrons increases.
This is because as we move from left to right in a period, electrons are added in same valence shell and effective nuclear charge (no. of protons in a shell) increases. So, attraction for the incoming electron increases.
-
Q10
Write the electronic configuration of two elements X and Y whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element X reacts with Y. Draw electron-dot structure of the product and also state the nature of the bond formed between both the elements.
Marks:3View AnswerAnswer:
Electronic configuration of element ‘X’ with atomic number 20 = 2,8,8,2
Electronic configuration of element ‘Y’ with atomic number 17 = 2,8,7
Number of valence electrons of X = 2
Valency of X = 2Number of valence electrons of Y = 7
Valency of Y = 8 - 7 = 1
Therefore, the formula of compound the compound is XY2.
Ionic bond will be formed between element X and Y. Element ‘X’ with atomic number 20 has electronic configuration 2,8,8,2. Thus it gives its two electrons to two atoms of Y and forms X2+ ions. While element ‘Y’ with atomic number 17 has electronic configuration 2,8,7. Thus two atoms of Y gain one e- each and forms 2Y– ions. So the electron dot structure for this divalent halide will be as follows—



