Science: 2017: CBSE: [Delhi]: Set – II
To Access the full content, Please Purchase
-
Q1
Write the molecular formula of first two members of homologous series having functional group – Br.
Marks:1View AnswerAnswer:
The molecular formula of first member is CH3Br and the molecular formula of second member is C2H5Br
-
Q2
How does Planaria reproduce? Is this method sexual or asexual?
Marks:1View AnswerAnswer:
Planariareproduces by regeneration. It is a type of asexual reproduction. In asexual reproduction, the planarian detaches its tail end and each half regrows the lost parts. If a Planaria is cut into two halves, both halves may become two new Planaria.
-
Q3
Why is forest considered a natural ecosystem?
Marks:1View AnswerAnswer:
A forest ecosystem is a natural ecosystem consisting of biotic components-all plants, animals and micro-organismsin in that area functioning together with abiotic components-all of the non-living physical factors of the environment.
-
Q4
An object is placed at a distance of 40 cm form a convex mirror of radius of curvature 40 cm. List four characteristics of the image formed by the mirror.
Marks:2View AnswerAnswer:
It is given that the object is placed somewhere between the pole and infinity. The ray diagram for the given case is as shown below:
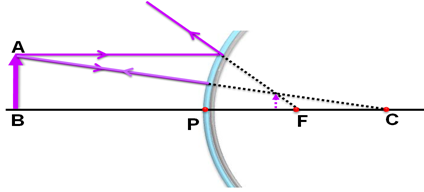
Four characteristics of the image formed by the mirror are:
(i) The image is formed behind the mirror.
(ii) The image formed is virtual.
(iii) The image formed is erect.
(iv) The image formed is diminished.
-
Q5
Why is sustainable management of natural resources necessary? Why is reuse better as compared to recycle?
Marks:2View AnswerAnswer:
Sustainable management of natural resources is necessary because:
i. It helps in the conservation of a replenishable resource, which otherwise may get depleted soon.
ii. Depletion of one resource may affect other resources also.
iii. It also prevents pollution and maintains our ecosystem.
Re-use strategy is better than recycling because:i. Recycling uses some energy.
ii. Recycling needs segregation of wastes.
-
Q6
Explain how would the involvement of local people be useful for successful management of forests.
Marks:2View AnswerAnswer:
Active participation of local people to save forests is an activity towards the conservation of forests. There are many examples, which suggest that involvement of local communities is necessary for any conservation effort. The Bishnoi community of Rajasthan is one such example. Amrita Devi Bishnoi is still remembered with reverence for the way she fought for protecting the khejri trees in Khejrali village. In 1731, she along with 363 other people sacrificed her life for the protection of khejri trees.
-
Q7
Complete the following chemical equations:
(i) CH3COOH + Na2CO3
(ii) CH4 + O2
(iii) C2H5OH + Na
Marks:3View AnswerAnswer:
-
Q8
Two carbon compounds X and Y have the molecular formula C3H6 and C4H10 respectively. Which one of the two is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case.
Marks:3View AnswerAnswer:
The general formula of alkenes is CnH2n. Therefore, the molecular formula C3H6 represents an alkene.
The general formula of alkanes is CnH2n+2. Therefore, the molecular formula C4H10 represents an alkane.
Alkane is a saturated hydrocarbon whereas alkene is an unsaturated hydrocarbon. Since, addition reactions are characteristic of alkenes. Therefore, C3H6 is likely to show addition reactions. The reaction in which an unsaturated hydrocarbon combines with another substance to give a single product is called an addition reaction. It is a characteristic property of unsaturated hydrocarbons (alkenes and alkynes).
The unsaturated compounds have multiple bonds. During the addition reactions, the addition takes place at multiple bond and new single bonds are formed.
-
Q9
An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reason:
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.
Marks:3View AnswerAnswer:
(i) Positions in Periodic Table:
Electronic configuration of element with atomic number 20 is 2, 8, 8, 2. According to its configuration, P belongs to 2nd group because it has 2 electrons in its outermost shell and 4th period of the modern periodic table as the element is of N electronic shell.
On the other hand, electronic configuration of element ‘Q’ with atomic number 17 is 2, 8, 7. According to its configuration, Q belongs to 17th group because it has 7 electrons in its outermost shell and 3rd period of the modern periodic table as the element is of M electronic shell.
(ii) Molecular formula of compound formed:
Atomic number of P = 20
Electronic configuration of P = 2,8,8,2
Number of valence electrons of P = 2
Valency of P = 2Atomic number of Q= 17
Electronic configuration of Q = 2,8,7
Number of valence electrons of Q = 7
Valency of Q = 8 - 7 = 1
Therefore the formula of the compound formed is PQ2. -
Q10
Write the names given to the vertical columns and horizontal rows in the Modern Periodic Table. How does the metallic character of elements vary on moving down in a vertical column? How does the size of the atomic radius vary on moving left to right in a horizontal row? Give reason in support of your answer in the above two cases.
Marks:3View AnswerAnswer:
Modern Periodic Table has 18 vertical columns known as ‘Group’ and 7 horizontal rows are known as ‘Period’.
(a) On moving down in a vertical column metallic character increases because on moving down in a group atomic size increases due to addition of a new shell, therefore, it can easily lose electrons from its valence shell.
(b) On moving left to right in a horizontal row atomic size decreases because nuclear charge on valence shell electrons increases.



