Science: 2017: CBSE: [Delhi]: Set – III
To Access the full content, Please Purchase
-
Q1
Write the molecular formula of first two members of homologous series having functional group-OH.
Marks:1View AnswerAnswer:
The molecular formula of first member is CH3OH and the molecular formula of second member is C2H5 OH.
-
Q2
How does Plasmodium reproduce? Is this method sexual or asexual?
Marks:1View AnswerAnswer:
Plasmodium reproduces asexually through the process of multiple-fission. In this method, repeated division of the parent nucleus is followed by division into many daughter cells.
-
Q3
Why is a lake considered to be a natural ecosystem?
Marks:1View AnswerAnswer:
A lake ecosystem is a natural ecosystem consisting of biotic components - all plants, animals and micro-organismsin that area functioning together with abiotic components - all of the non-living physical factors of the environment.
-
Q4
An object is placed at a distance of 12 cm in front of a concave mirror of radius of curvature 30 cm. List four characteristics of the image formed by the mirror.
Marks:2View AnswerAnswer:
Focal length = radius of curvature/2
= 30 cm/2 = 15 cm
Thus, the object is placed between the pole and the focus of the concave mirror. The ray diagram in this case is as shown below:
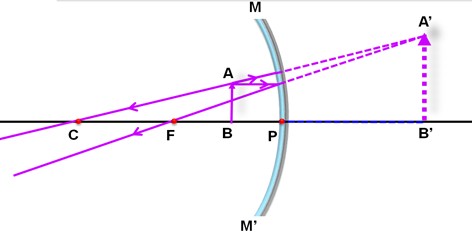
Four characteristics of the image formed by the mirror are:
(i) The image is formed behind the mirror.
(ii) The image formed is virtual.
(iii) The image formed is erect.
(iv) The image formed is enlarged.
-
Q5
How do advantages of exploiting natural resources with short term gains in mind differ from the advantages of managing our resources with a long-term perspective?
Marks:2View AnswerAnswer:
Natural resources are limited in nature so we must aim at adopting steps in daily life for conservation of natural resources. For long term perspective we must aim at judicious use of natural resources as they are limited in nature and we should conserve them for future generation. We should use natural resources in judicious and ecofriendly manner to ensure equal distribution among the people.
-
Q6
What is meant by wildlife? How is it important for us?
Marks:2View AnswerAnswer:
Wildlife is defined as the biota including both, animals and plants that grow independentlyof people, usually in the natural conditions. It includes all the non-domesticated and non-cultivated flora and fauna of a geographic region.
Wildlife is important as it plays an essential role in the functioning of the food webs and the food chains and thus maintaining the ecological balance of an ecosystem. Not just this, it is also important for the maintenance of aesthetic, educational, historical and scientific values, for maintaining the genetic diversity.All crops and domestic animals have descended from their wild varieties and many wildlife species yield products of immense medicinal value.
-
Q7
Complete the following chemical reactions
Marks:3View AnswerAnswer:
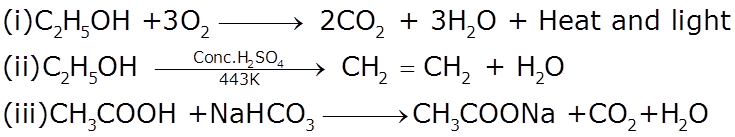
-
Q8
The molecular formula of two carbon compounds are C4H8 and C3H8. Which one of the two is most likely to addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case.
Marks:3View AnswerAnswer:
The general formula of alkenes is CnH2n. Therefore, the molecular formula C4H8 represents an alkene
The general formula of alkanes is CnH2n+2. Therefore, the molecular formula C3H8 represents an alkane.
Alkane is a saturated hydrocarbon whereas alkene is an unsaturated hydrocarbon. Since, addition reactions are characteristic of alkenes. Therefore, C4H8 is likely to show addition reactions. The reaction in which an unsaturated hydrocarbon combines with another substance to give a single product is called an addition reaction. It is a characteristic property of unsaturated hydrocarbons (alkenes and alkynes).
The unsaturated compounds have multiple bonds. During the addition reactions, the addition takes place at multiple bond and new single bonds are formed.
-
Q9
Write the names given to the vertical columns and horizontal rows in the Modern Periodic Table. How does the metallic character of elements vary on moving down in a vertical column? How does the size of the atomic radius vary on moving left to right in a horizontal row? Give reason in support of your answer in the above two cases.
Marks:3View AnswerAnswer:
Modern Periodic Table has 18 vertical columns known as ‘Group’ and 7 horizontal rows are known as ‘Period’.
(a) On moving down in a vertical column metallic character increases because on moving down in a group atomic size increases due to addition of a new shell, therefore, it can easily lose electrons from its valence shell.
(b) On moving left to right in a horizontal row atomic size decreases because nuclear charge on valence shell electrons increases.
-
Q10
An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reason:
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.
Marks:3View AnswerAnswer:
(i) Positions in Periodic Table:
Electronic configuration of element with atomic number 20 is 2, 8, 8, 2. According to its configuration, P belongs to 2nd group because it has 2 electrons in its outermost shell and 4th period of the modern periodic table as the element is of N electronic shell.
On the other hand, electronic configuration of element ‘Q’ with atomic number 17 is 2, 8, 7. According to its configuration, Q belongs to 17th group because it has 7 electrons in its outermost shell and 3rd period of the modern periodic table as the element is of M electronic shell.
(ii) Molecular formula of compound formed:
Atomic number of P = 20
Electronic configuration of P = 2,8,8,2
Number of valence electrons of P = 2
Valency of P = 2Atomic number of Q= 17
Electronic configuration of Q = 2,8,7
Number of valence electrons of Q = 7
Valency of Q = 8 - 7 = 1
Therefore the formula of the compound formed is PQ2.



