Science:2019:CBSE:[Delhi]: Set-II
To Access the full content, Please Purchase
-
Q1
Name and define the SI unit of current.
Marks:2View AnswerAnswer:
The SI unit of electric current is Ampere.
An electric current is said to be one ampere, if one coulomb of electric charge flows through a given area of cross section of a conductor in one second. -
Q2
Write the name of the main constituent of biogas. Also state its percentage.
Marks:1View AnswerAnswer:
The main constituent of biogas is methane. Biogas contains up to 75% of methane.
-
Q3
Write the name, symbol and electronic configuration of an element X whose atomic number is 11.
Marks:2View AnswerAnswer:
Element X with atomic number 11 is sodium and its symbol is Na.
Electronic configuration of X is 2, 8, 1. -
Q4
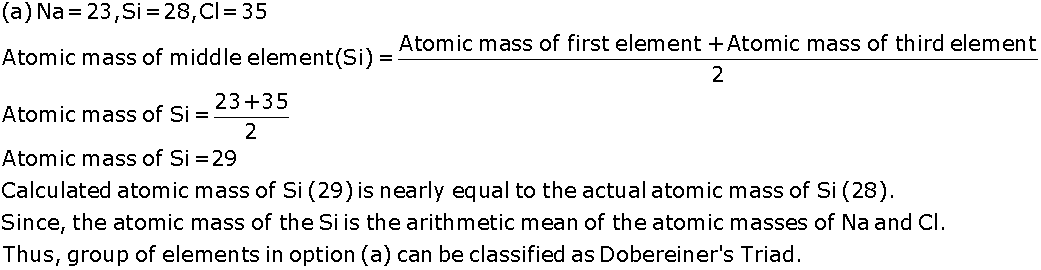
Can the following groups of elements be classified as Dobereiner's triad?
(a) Na, Si, Cl (b) Be, Mg, Ca
(Atomic mass of Be-9, Na-23, Mg-24, Si-28, Cl-35, Ca-40)
Justify your answer in each case.
Marks:2View AnswerAnswer:
According to the Dobereiner's law of triads, the atomic mass of the middle element of a triad is the arithmetic mean of the atomic masses of the other two elements.

-
Q5
How is O2 and CO2 transported in human beings?
Marks:2View AnswerAnswer:
In human beings, oxygen (O2) and carbon dioxide (CO2) is transported in human beings with the help of a respiratory pigment called haemoglobin. This pigment is present in the red blood cells. During respiration diffusion of oxygen into the blood occurs in the capillaries of the alveoli. The haemoglobin has a high affinity for oxygen. In alveoli, haemoglobin takes up oxygen from the air in the alveoli and transports it to various organs, tissues or cells of the body. In the cell, carbon dioxide moves out of the cell by the process of diffusion and is is transported from the body cell to the lungs in the forms of carboxy-haemoglobin, carbonic acid, bicarbonates of sodium and potassium.
-
Q6
Write the structure of eye lens and state the role of ciliary muscles in the human eye.
Marks:2View AnswerAnswer:
Eye lens is a convex lens. It is a transparent, flexible jelly like material made up of proteins.
The main role of the ciliary muscles is to change the curvature and focal length of the eye lens to focus on near and far objects accordingly. -
Q7
Identify the acid and base which form sodium hydrogen carbonate. Write chemical equation in support of your answer. State whether this compound is acidic, basic or neutral. Also write its pH value.
Marks:3View AnswerAnswer:

-
Q8
Based on the group valency of elements write the molecular formula of the following compounds giving justification for each:
(i) Oxide of first group elements.
(ii) Halides of the elements of group thirteen,
(iii) Compound formed when an element, A of group-2 combines with an element, B of group seventeen.
Marks:3View AnswerAnswer:
(i) The formula of oxide of first group elements will be M2O. Where M represents group 1 element like Li, Na, K etc. The valency of group 1 elements is one as they have one electron in the valence shell whereas valency of oxygen is 2. To complete the octet of oxygen atom, two atoms of group 1 element combine with oxygen; hence the general formula of oxide of group 1 elements is M2O. Li2O, Na2O and K2O are the some of the oxides of group 1 elements.
(ii) The formula of halides of group 13 elements is MX3.
Where, M= group 13 elements (B, Al) and X= group 17 elements (Cl, Br, I).
The elements of group 13 have three electrons in the valence shell and their valency is 3. Halogens have 7 valence electrons and their valency is 1. To complete the octet of group 13 elements, three atoms of halogens share 1 electron each, hence the general formula of halide of group 13 elements is MX3.
(iii) The formula of compound when an element A of group–2 combines with an element B of group–17 is AB2. The elements of group–2 have 2 electrons in the valence shell and their valency is 2. To complete the octet of group 2 elements, two atoms of group–17 share 1 electron each, hence the general formula of compound is AB2.
-
Q9
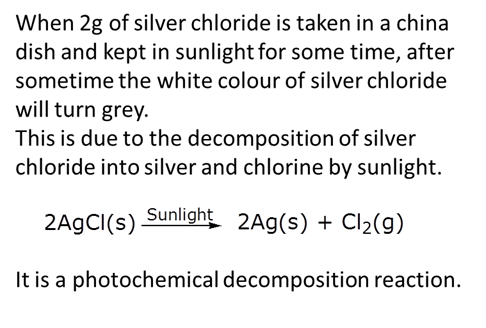
2g of silver chloride is taken in a china dish and the china dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction.
Marks:3View AnswerAnswer:
-
Q10
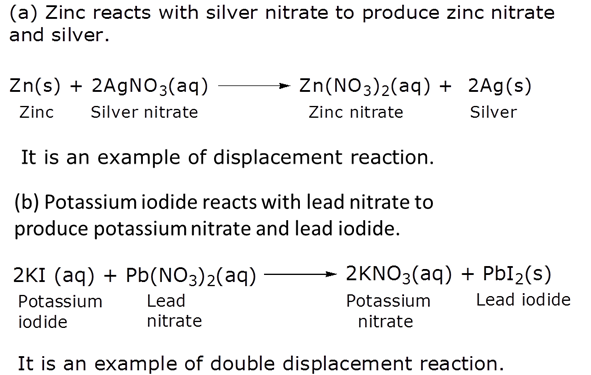
Identify the type or reactions taking place in each of the following cases and write the balanced chemical equation for the reactions.
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(b) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
Marks:3View AnswerAnswer: