Chemistry:2013:CBSE:[Delhi]:Set-I
To Access the full content, Please Purchase
-
Q1
How many atoms constitute one unit cell of a face-centred cubic crystal?
Marks:1View AnswerAnswer:

-
Q2
Name the method used for the refining of the nickel metal.
Marks:1View AnswerAnswer:
Mond’s process is used for refining of nickel.
-
Q3
What is the covalency of nitrogen in N2O5?
Marks:1View AnswerAnswer:
The covalency of the nitrogen depends upon the number of shared pair of electrons. In N2O5, each nitrogen atom has four shared pair of electrons. Therefore, the covalency of the nitrogen in N2O5 is 4.
-
Q4
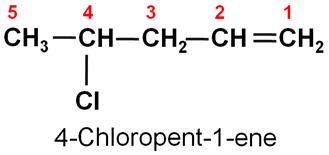
Write the IUPAC name of
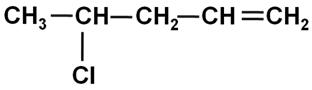 Marks:1View Answer
Marks:1View AnswerAnswer:
-
Q5
What happens when CH3–Br is treated with KCN?
Marks:1View AnswerAnswer:

-
Q6
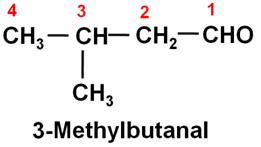
Write the structure of 3-methylbutanal.
Marks:1View AnswerAnswer:
-
Q7
Arrange the following in the increasing order of their basic strength in the aqueous solution:
CH3NH2, (CH3)3N, (CH3)2NHMarks:1View AnswerAnswer:
The increasing order of the basic strength of methylamines in the aqueous solution is as follows:
(CH3)3N < CH3NH2 < (CH3)2NH -
Q8
What are three types of RNA molecules that perform different functions.
Marks:1View AnswerAnswer:
There are the three different types of RNA molecules:
1. Messenger RNA (m-RNA)
2. Transfer RNA (t-RNA)
3. Ribosomal RNA (r-RNA) -
Q9
18 g of glucose, C6H12O6 (Molar Mass = 180 g mol–1) is dissolved in 1 kg of water in a sauce pan. At what temperature will this solution boil?
(Kb for water = 0.52 K kg mol–1, boiling point of pure water = 373.15 K)Marks:2View AnswerAnswer:
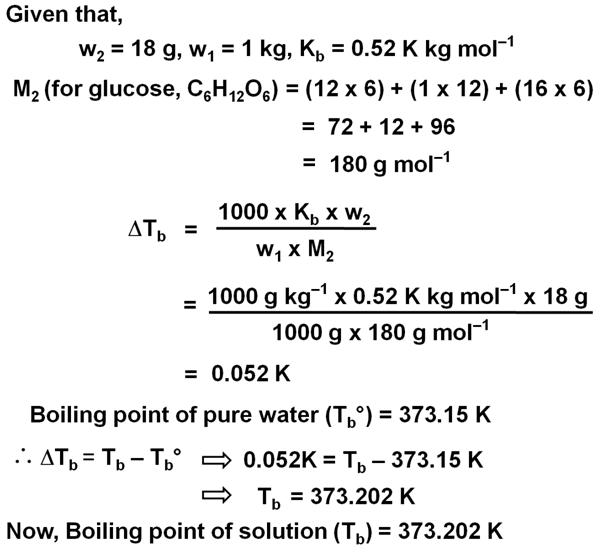
-
Q10
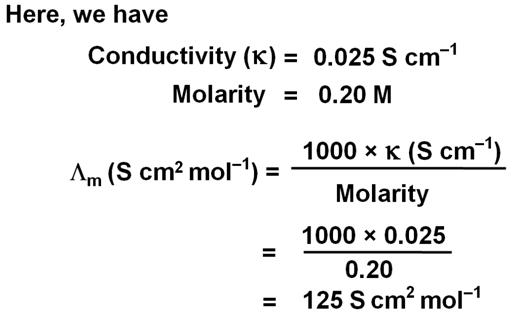
The conductivity of 0.20 M solution of KCl at 298 K is 0.025 S cm–1. Calculate its molar conductivity.
Marks:2View AnswerAnswer: